Statement of
DR. JOHN T. EVERETT
HEARING ON
The Environmental and Economic Impacts of Ocean Acidification
BEFORE THE
Subcommittee on Oceans, Atmosphere, Fisheries, and Coast Guard of the
Committee on Commerce, Science, and Transportation
United States Senate
April 22, 2010
Mr. Chairman and Members of the
Committee, thank you for inviting me to appear before you today. I am John
Everett. I am not here to represent any particular organization, company, nor
special-interest group. I have never received any funding to support my climate
change work other than my NOAA (National Oceanic and Atmospheric
Administration) salary, from which I retired after a 31 – year career in
various positions. Thirty years ago I was loaned to this Committee for one year
and was responsible for oceans and fisheries issues and NOAA oversight. I was a
Member of the Board of Directors of the NOAA Climate Change Program from its
inception until I left NOAA. I led several impact analyses for the
Intergovernmental Panel on Climate Change from 1988 to 2000, while a NOAA
employee. The reports were reviewed by hundreds of government and academic
scientists as part of the IPCC process. My work included five impact analyses:
Fisheries (Convening Lead Author), Polar Regions (Co-Chair), Oceans (Lead
Author), and Oceans and Coastal Zones (Co-Chair/2 reports). Since leaving NOAA
I have kept abreast of the literature, have continued as an IPCC Expert
Reviewer, have talked to many individuals and groups and have maintained these
subjects in the UN Atlas of the Oceans, where I am the Chief Editor and Project
Manager. I own a fisheries and oceans consulting business called Ocean
Associates, Inc[1]. and a
website ClimateChangeFacts.Info[2]
that I try to keep unbiased in its treatment of conflicting science. This site
is the number 1 Google-ranked site of many million for certain climate search
terms. My approach to impact analysis is a product of my education and work
experiences at NOAA and the work I led for IPCC. This statement provides my
analysis of the effects of ocean acidification on our living resources and our
economy.
All opinions are mine alone.
I was assigned the climate change
duties when I was the NOAA National Marine Fisheries Service Division Chief for
Fisheries Development in the 1970s. The agency was very concerned about the
impact of climate change on the United States fisheries and fishing industry. Global cooling would be devastating to
our fisheries and aquaculture. About 1987, the momentum shifted to fears of global warming and with my background, and
as Director of Policy and Planning for NOAA Fisheries, I was tasked to lead our
efforts dealing with it. In 1996 I received the NOAA Administrator’s Award for
“accomplishments in assessing the impacts of climate change on global oceans
and fisheries.” In 2008, I received recognition
from the IPCC for having “contributed to the work of the IPCC over the years
since inception of the organization”, leading to its Nobel Peace Prize.
I. The Concerns
There are several concerns about CO2 entering the oceans and causing its pH to become lower. Their discussion in the press and among policy officials is at the foundation of this hearing. These concerns are:
1. Animals with calcium carbonate shells will lose the ability to make shells
2. Existing shells will become weaker
3. Loss of shell-forming animals will reduce food for those higher in the food chain
4. Many species will be gone in 30 years
5. Oysters and clams are dying
6. Jellyfish are increasing
7. Seagrasses will be injured.
The concerns are based on the work of respected scientists who have shared the above beliefs or authored papers that argue the above points. They believe increased atmospheric CO2 will increase the acidification of the oceans. The basis is largely a set of emission scenarios developed by IPCC in the early 1990s in an attempt to reign in the mass confusion about the future trajectory of CO2 emissions. With this standard set of scenarios, climate modelers could then have a standard set of inputs in terms of what was broadly considered a primary determinant of climate – the proportion of CO2 in the atmosphere. This proportion is based on new contributions after deducting removals by the Earth system and assumes a decreasing removal ability as CO2 increases. For the first time, modelers around the world could compare results while impact assessment scientists and policy makers could look at points on which most models agreed. Standardization of scenarios allowed modelers to identify errors or alternative ways to predict or handle parameters, such as cloud cover. One of the scenarios became heavily used and is identified as IS92 – Business as Usual. Nearly 20 years ago, it was a reasonable approach and pretty much in the middle range of alternative scenarios. It underpins much of the research findings I will present today.
There are other respected scientists who believe that the Business as Usual scenario has been overtaken by events. The cost of fossil fuels is rising, reflecting increasing scarcity and contributing to a slower CO2 growth in the atmosphere and a lack of acceleration. New science shows the Earth’s ability to absorb the same proportion of new CO2 each year has not been diminished, removing a key underlying assumption. Importantly, oceans are alkaline - not acidic (much more so than rain-water), so use of the term “acidification” promotes fear. If all the CO2 in the air were put into the ocean, the oceans would still be alkaline. With all this talk of acidification, we need to reassure bathers that their feet will not dissolve when they step into the ocean. Ocean water at the surface generally has a pH over 8 and neutral is 7.0 (pure water) while a puddle of rain water is 100 times more acidic after having picked up CO2 in its fall. Technically, we should say the oceans could become less alkaline, rather than more acidic. In any case, unlike rainwater, the oceans will never become acidic.
At the bottom of our inverted pyramid of climate science are a few good scientists working to improve our knowledge of how the Earth system operates, and then to project future possibilities. The physics are daunting. Similarly, the modelers must get observational input data from the physical world and from prognosticators about how many people will be born in future years and how they will get and use their energy. The number of scientists doing this work is small compared to the number who will use their information to analyze impacts and make policy recommendations to governments and industry.
As a research manager much of my life, I have a healthy skepticism of things that underpin important decisions. Whether it is a column of numbers that will tie up a fishing fleet because of an addition error or a wiring harness on a manned lunar rocket that doesn’t quite fit, I have learned to pause and check it out. There are some things at the bottom of the CO2 pyramid that make it seem wobbly and in need of a check.
Physics tells us that increasing atmospheric CO2 lowers oceanic pH and carbonate ion concentrations, thereby decreasing calcium carbonate. Surface ocean pH today is believed to be 0.1 unit lower than pre-industrial values. (See footnote on pH[3].) The median value of ocean model runs projects that pH will decrease by another 0.3 to 0.4 units by 2100. This translates into a 100 to 150% increase in the concentration of H+ ions while carbonate ion concentrations will decrease. When water is undersaturated with respect to calcium carbonate, marine organisms can no longer form calcium carbonate shells, if the living shell is directly exposed to the water. The model simulations project that undersaturation will be reached in a few decades[1]. The conventional wisdom also says that as CO2 concentration becomes higher, saturation will mean that more of it will remain in the atmosphere each year, accelerating its accumulation.
However there are some major problems with the science. The wisdom at the time of the IPCC 2007 report was that half of CO2 emissions would remain in the atmosphere and that we would have 712 ppm (IS92a) by 2100[2]. This would require the atmosphere to more than double the present rate of growth of CO2 to 3.05 ppm, yet the growth rate seems to be leveling off. The meaning of this information (and the future of all climate models based on it) became VERY cloudy on 31 December 2009 with the ScienceDaily acknowledgment of a paper published by American Geophysical Union and authored by Wolfgang Knorr that shows "No Rise of Atmospheric Carbon Dioxide Fraction in Past 160 Years", despite the predictions of carbon cycle/climate models[3]. The implications of this have yet to be assimilated by the modeling community. This does not mean that CO2 proportion is not rising but rather that the proportion not being assimilated has not changed since 1850. Importantly, it means that the rate of CO2 cycling increases as it becomes more concentrated, and does not decrease as assumed in climate models. The rate of projected growth in CO2 appears to be greatly exaggerated.
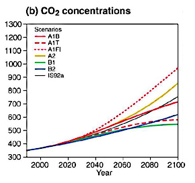 The CO2 scenarios are literally falling flat and need revision.
The observational trend line shows monotonic growth – pretty much a
straight line as in the chart below of global marine CO2 measurements (NOAA
data)[4], while the IPCC scenarios used in most
research rely on an accelerating growth. Certainly the predicted rapid acceleration
of the IS92a model (see solid black line in middle of figure) is missing from
the NOAA data plotted below. In fact, if we wonder if the last 8 or 12 years
are representative of the future, we might imagine a downward slope in the
growth rate. This could be real as rising prices cut usage and lead to economic
distress. It could also mean that the ocean is absorbing more CO2, which might
not bode well in light of concerns over acidification. However, it may be that
the ocean is converting and storing the CO2 as calcium carbonate in the form of
shells of oyster, clams and planktonic organisms. It is a complicated
environment and there is much we do not know.
The CO2 scenarios are literally falling flat and need revision.
The observational trend line shows monotonic growth – pretty much a
straight line as in the chart below of global marine CO2 measurements (NOAA
data)[4], while the IPCC scenarios used in most
research rely on an accelerating growth. Certainly the predicted rapid acceleration
of the IS92a model (see solid black line in middle of figure) is missing from
the NOAA data plotted below. In fact, if we wonder if the last 8 or 12 years
are representative of the future, we might imagine a downward slope in the
growth rate. This could be real as rising prices cut usage and lead to economic
distress. It could also mean that the ocean is absorbing more CO2, which might
not bode well in light of concerns over acidification. However, it may be that
the ocean is converting and storing the CO2 as calcium carbonate in the form of
shells of oyster, clams and planktonic organisms. It is a complicated
environment and there is much we do not know.
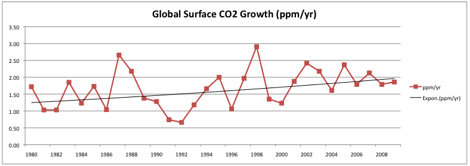
Using the average rate of increase for the past 10 years (1.87/year), and assuming a straight-line growth, my projection for 2100 is 560 ppm. I have great reservations about our ability to find the necessary amount of fuel even this would require, never mind enough to reach 712 ppm (IS92a) or higher.
Thus, if the projections we are concerned with today are based on the IPCC IS92a model, or one of its cohorts, and the concept of CO2 sink saturation, we should give the information on its impacts a second look.
Further, if a model can’t replicate the past by relying on principles of physics and mathematics, without “tuning” its parameters to reflect past variations, we must not trust that it properly represents the real world. Some important physics may be missing or misrepresented. This is particularly true of any model that failed to predict the present leveling of temperatures in the face of rising CO2. I know of none that got it right.
Something is very wrong at the bottom of our inverted pyramid!
III. The Biology
The Concerns
Much of the concern flows form the latest IPCC report. The text from the Summary for Policy Makers states: “The uptake of anthropogenic carbon since 1750 has led to the ocean becoming more acidic with an average decrease in pH of 0.1 units. Increasing atmospheric CO2 concentrations lead to further acidification. Projections based on SRES scenarios give a reduction in average global surface ocean pH of between 0.14 and 0.35 units over the 21st century. While the effects of observed ocean acidification on the marine biosphere are as yet undocumented, the progressive acidification of oceans is expected to have negative impacts on marine shell-forming organisms (e.g. corals) and their dependent species”[5]
1. Animals and plants with calcium carbonate “shells” will lose the ability to make shells. These animals include corals, coralline algae (e.g., encrusting algae), and foraminifera, pteropods (swimming planktonic snails with aragonite shells), and mollusks (e,g,. clams and oysters).
2. Existing shells will become weaker and even dissolve. Dissolution of shells after death is the norm. Calcium carbonate flows back into the water wherever it is not saturated. In the deep ocean, this can happen rapidly to exposed shells.
3. Loss of shell-forming animals will reduce food for those higher in the food chain. Dissolved calcium and carbonate ions are used by ocean animals to produce their shells and skeleton. A lower pH can slow shell production by disrupting the supply of carbonate ions, thus slowing shell production and increasing the susceptibility to dissolution, early death and predation.
4. Many species will be gone in 30 years. This is founded in a belief in the IS92a emission scenarios and some research results.
5. Oysters and clams are dying. In the Pacific Northwest there are charges that an acidic ocean is to blame for extensive mortalities of young oysters and clams. Fears include the possibility that acidic upwelling waters will get even more so from the infusion of high CO2 air.
6. Jellyfish are increasing. Some have postulated that ocean acidification could open ecological space for noncalcifying species.
7. Seagrasses will be injured. Acid waters will disrupt life processes and slow growth.
Biological considerations
There is limited research. I have reviewed the major papers and the critiques of the papers. Below are a few that I think merit bringing before the committee. It is only a few that show no obvious bias. For example, it is quite common among researchers vying for scarce funding dollars to hype their findings or the importance of the problem. Whether it is the use of hydrochloric (HCl) acid to mimic CO2 but which introduces other issues such as shell decay, or presenting the findings of grave consequences at high acidity while not mentioning the lack of change at lower levels, or not investigating whether low pH was due to degraded water quality from runoff and sewage, the real cause of reduced growth or mortality. In some cases a lower base year is chosen that exaggerates the percentage change, such as “pH levels will drop 30% from pre-industrial levels – when current levels are far less disputed, but the % change is less.
Each study must be scoured for hints of inappropriate procedures and unfounded statements. None can be accepted at face value. The peer review process has warts. A good example is the dispute over whether acidification is good or bad for “shell”-forming plant plankton, a vital part of the ocean’s biology with the ability to sequester vast amounts of CO2. The first paper says more CO2 is good, the second that it is bad, and then the first successfully refutes the criticism and gets the last word, sustaining the positive assessment in great detail - all published in Science.
“Ocean acidification in response to rising atmospheric CO2 partial pressures is widely expected to reduce calcification by marine organisms. From the mid-Mesozoic, coccolithophores have been major calcium carbonate producers in the world’s oceans, today accounting for about a third of the total marine CaCO3 production. Here, we present laboratory evidence that calcification and net primary production in the coccolithophore species Emiliania huxleyi are significantly increased by high CO2 partial pressures. Field evidence from the deep ocean is consistent with these laboratory conclusions, indicating that over the past 220 years there has been a 40% increase in average coccolith mass. Our findings show that coccolithophores are already responding and will probably continue to respond to rising atmospheric CO2 partial pressures, which has important implications for biogeochemical modeling of future oceans and climate.[6] ”However, Riebesell et al. vigorously attacked the paper, claiming that “shortcomings in their experimental protocol compromise the interpretation of their data and the resulting conclusions.”[7] In rebuttal, also in Science, Iglesias-Rodriguez et al. get the last word by successfully demonstrating that the logic and methods of Riebesall et al. are the ones that are flawed and the original findings of increased calcification are valid[8].
 Perhaps the most thorough review of the
literature on acidificaton impacts is by Fabry et al.[9] They found that little research was done on
CO2 concentrations that were relevant to answer today’s questions. They express
much concern that acidification will retard development of shells. They, as do
several other authors, note that studies have not been long-term enough to
discover adaptations over multiple generations. I believe this is key because
these genera have genetic information about past events and this may well take
several generations for stabilization. In any scenario, there will be ample
time for this to happen. In a laboratory it happens with the throw of a switch.
If my family or its descendants needs to hold its head underwater for 4 minutes
and they have a couple generations to adapt, it can be done. However, I can’t
do it very well today.
Perhaps the most thorough review of the
literature on acidificaton impacts is by Fabry et al.[9] They found that little research was done on
CO2 concentrations that were relevant to answer today’s questions. They express
much concern that acidification will retard development of shells. They, as do
several other authors, note that studies have not been long-term enough to
discover adaptations over multiple generations. I believe this is key because
these genera have genetic information about past events and this may well take
several generations for stabilization. In any scenario, there will be ample
time for this to happen. In a laboratory it happens with the throw of a switch.
If my family or its descendants needs to hold its head underwater for 4 minutes
and they have a couple generations to adapt, it can be done. However, I can’t
do it very well today.
With respect to corals, Atkinson reviewed recent literature on ….“how ocean acidification may influence coral reef organisms and coral reef communities. We argue that it is unclear as to how, and to what extent, ocean acidification will influence calcium carbonate calcification and dissolution, and affect changes in community structure of present-day coral reefs[10]”. Also, the latest IPCC report (summary above) found no empirical evidence supporting effects of acidification on marine biological systems[11].
Kurihara et al investigated the “effects of seawater equilibrated with CO2-enriched air (2000 ppm, pH 7.4) on the early development of the mussel” and found that the mussels, as clams studied by them earlier, were significantly impaired when exposed to CO2 over 5X! that of today[12].
Marubini et al. found that seawater acidification may lead to a decrease of tropical coral growth calcification. This effect is either mediated by a decrease in carbonate, in pH, or by an alteration of the internal buffering system leading to a disruption of carbon supply to calcification rather than by a direct effect of CO2 or a change of HCO3 - concentration. Results showed that the negative effect of acidification may be counteracted by increasing the bicarbonate concentration of seawater, resulting in an increase in the carbonate concentration.[13]
Research in laboratories shows that shell growth is slowed in some animals and enhanced in others. Woods Hole Oceanographic Institution (WHOI) researchers found that 7 of 18 species of animals “such as crabs, shrimp and lobsters—unexpectedly build more shell when exposed to ocean acidification caused by elevated levels of atmospheric carbon dioxide (CO2)”S[14]. They tested as high as 7 times present levels. They found that hard clams and corals slowed formation of shells but only above 1,000 ppm, while soft clams and oyster slowed formation at lower levels. Note that the shells did not dissolve, but only grew somewhat slower at 7X! present CO2 concentrations.
There is no basis to predict the demise of shelled animals living in the sea or the animals above them in the food chain at any likely level of CO2 that might be put in the air by humans.
With respect to the homing ability of fish, a study at the University of Hawaii found the olfactory-based homing ability of clownfish was disrupted at 1,000 ppm and non-existent at 2,000 ppm. The values of CO2 acidification were high: “These values are consistent with climate change models that predict atmospheric CO2 levels could exceed 1,000 ppm by 2100 and approach 2,000 ppm by the end of next century under a business as usual scenario”[15] This has implication for all fish that need to find their way back to natal streams, if we were ever to get to 1,000 ppm.
With respect to clam and oyster mortalities being caused by acidified water, it is unlikely that CO2 deposition from the air is the culprit. Upwelling brings water from the depths to the surface. This water has been out of sunlight perhaps for centuries. There has been no photosynthesis for plants to turn the CO2 into oxygen, and whatever oxygen there was, has been converted into CO2 by animals. When this cold water reaches the surface, it is saturated with CO2 and is relatively acidic (but still alkaline), plus it has little oxygen. As this water warms, it will be outgassing CO2, rather than picking it up as claimed by some. Less alkaline water is also symptomatic of coastal eutrophication, whether caused by runoff or sewage. The WHOI work cited above shows that the growth of clams and oysters can be slowed by CO2-induced acidification. In their studies, the animals did not die even at rates several multiples of today’s CO2 levels and for clams, growth slowed only at the highest levels of CO2.
With respect to being overrun with jellyfish, some have suggested this will happen because ocean acidification could open ecological space for noncalcifying species. Richardson and Gibson studied the possibility that there were more noncalcifying jellyfish when conditions were more acidic (lower pH) in the Northeast Atlantic using coelenterate records from the Continuous Plankton Recorder and pH data from the International Council for the Exploration of the Sea for the period 1946–2003. They could find no significant relationships between jellyfish abundance and acidic conditions in any of the regions investigated[16].
With respect to sea grasses, Zimmerman studied sea-grasses that form the bases of highly productive ecosystems ranging from tropical to polar seas. Despite clear evidence for carbon limitation of photosynthesis, seagrasses thrive in high light environments, and show little evidence of light-induced photoinhibition. Increasing the availability of dissolved aqueous CO2 can increase instantaneous rates of light saturated photosynthesis by up to 4 fold. Prolonged exposure to elevated CO2 concentrations increases the concentrations of non-structural carbohydrates (sucrose and starch), rates of vegetative shoot proliferation, and flowering, and reduces light requirements for plant survival. Consequently, seagrass populations are likely to respond positively to CO2-induced acidification of the coastal ocean, which may have significant implications for carbon dynamics in shallow water habitats and for the restoration/preservation of seagrass populations.[17]
IV. Has this happened before?
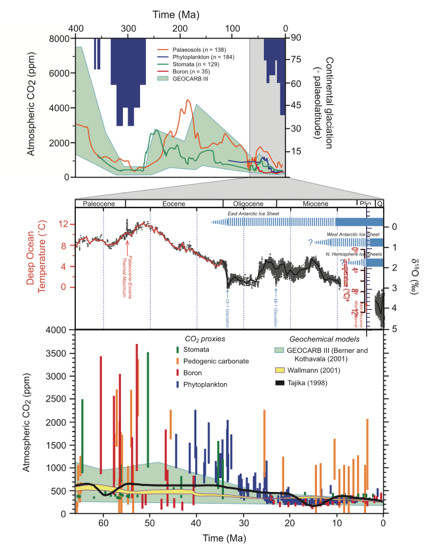 From 50-600 million years ago, atmospheric CO2 levels were
usually 2-20 times higher than at present. All the animals of concern evolved
during this period. This included the age of the dinosaurs, when life was so
prolific that we still use the carbon, limestone and chalk from those periods.
The animals of concern all should have the innate genetic plasticity to quickly
respond to the relatively modest changes of even the most unlikely scenarios,
none of which approach the earlier range. Most CO2 emissions originally came
from it during epochs when the species of concern flourished. The chart below
compiles the work of several authors and methods. It is from the latest IPCC
report, showing time in Ma (millions of years) before present. For comparison,
the present CO2 level is 388 ppm.
From 50-600 million years ago, atmospheric CO2 levels were
usually 2-20 times higher than at present. All the animals of concern evolved
during this period. This included the age of the dinosaurs, when life was so
prolific that we still use the carbon, limestone and chalk from those periods.
The animals of concern all should have the innate genetic plasticity to quickly
respond to the relatively modest changes of even the most unlikely scenarios,
none of which approach the earlier range. Most CO2 emissions originally came
from it during epochs when the species of concern flourished. The chart below
compiles the work of several authors and methods. It is from the latest IPCC
report, showing time in Ma (millions of years) before present. For comparison,
the present CO2 level is 388 ppm.
V. Is this bad or good or just different?
We and all other animals use oxygen and expel CO2. Plants do the opposite. CO2, combined with light and nutrients is their food. We must not lose sight of the fact that plants have consumed once-abundant CO2 to the point that it is 0.000388 of the atmosphere. Many greenhouse operators pump CO2 into their buildings to enhance growth, indicating plants evolved during higher concentrations of CO2. Plants in the ocean also rely on CO2. There is a high ability to move the excess out of circulation, turning it into oxygen (by plants) or calcium carbonate (by plants and animals). A view of the CO2 growth chart (above) and analyses such as that of Wolfgang Knorr cited above show this has not been adequately taken into account by climate modelers or those who provided their inputs.
We know that the Earth has seen these conditions before, and that all the same types of animals and plants of the oceans successfully made it through far more extreme conditions. Virtually all the ecological niches were filled at all times. If someone could demonstrate that there were no corals, clams, oysters, or shelled plankton when the Earth had double or triple the amount of CO2 in the air, we would have reason for concern. Just as IPCC has concluded, there is no observational evidence that things would be better or worse, or even different. Similarly, there is nothing conclusive in the very recent scientific literature to indicate any reason for concern. If anything, the science indicates plants, at least, will be more successful, and since they are the bottom of the food chain, this cannot be totally bad.
VI. What can be done about it?
Oceans are actually alkaline with a surface pH of around 8.1. But it can vary from higher levels in shallow areas, where CO2 and hydrogen ions are consumed by plants, to relatively acidic areas in eutrophic estuaries. Upwelling areas are also less alkaline, as cold bottom waters are brought into sunlight near the surface where algae use the deep-water CO2 and nutrients to create a productivity boom that sustains fisheries production in several areas of the world. There are no long-term data, using similar instruments that provide a real clue as to global trends in alkalinity. There are only a few data sets of over a decade, such as that of the Monterey Bay Aquarium. The variability, because of nearby ocean currents and upwelling shows the difficulty in portraying a global average value.
Some pundits have argued that we could add limestone to the oceans to make them more alkaline, but this has little merit due to costs and the fact that the oceans already contain immense buffering capability. We should bear in mind that this limestone and chalk for the most part came from the shells of plankton, as they fed on the CO2-laden ancient seas.
VII. research Suggestions
There are some items that would go a long way toward establishing the likely effects of an increased CO2 world.
1. Develop a CO2/temperature timeline based on extant research on past climates, at least back to about 600 million years before the present. This effort would provide a critical review of candidate papers and unpublished work that goes well beyond a typical peer-reviewed journal publication, or prior summary reports of the IPCC.
2. The acidification debate has showed us we lack a sufficient understanding of some fundamental chemical and biological processes. The research to resolve these questions should continue and perhaps be centrally coordinated internationally so that scarce dollars are targeted at real and important knowledge gaps.
3. Examine the growth rates, densities, and shell thicknesses of clams, oysters, or other mollusks from Indian middens and sediments to determine if any changes can be detected and if they correlate to any known changes in the oceans or atmosphere, including pH and CO2 levels.
4. Before the next IPCC assessment begins, assemble a USA review team and nominees for the IPCC writing and Chair assignments that make up a cross-section of scientific view-points. There are qualified scientists in agencies, industry, and among the citizenry who can contribute. Just as we shouldn’t have too many from the energy industry, the same goes for the agencies, universities, and NGOs. We all have biases, even if we think it is the other person who is the one with an agenda. We cannot afford to have only people with the same agenda, no matter how righteous they might think it to be.
VIII. Concluding Remarks
There is no reliable observational evidence of negative trends that can be traced definitively to lowered pH of the water. If there were, it would be suspect because there is insignificant change relative to past climates of the Earth. Scientific studies, and papers reviewing science studies, have similar messages. Papers that herald findings that show negative impacts need to be dismissed if they used acids rather than CO2 to reduce alkalinity, if they simulated CO2 values beyond triple those of today, while not reporting results at concentrations of half, present, double and triple, or as pointed out in several studies, they did not investigate adaptations over many generations. If there are reports of increases in ocean acidification in a region, the likely causes are upwelling, pollution, and rainfall (or runoff) and these all need to be addressed.
The oceans and coastal zones have been far warmer and colder and much more acidic than is projected by climate models. Marine life has been in the oceans nearly since when they were formed. During the millennia life endured and responded to CO2 levels well beyond anything projected, with temperature changes that put tropical plants at the poles or had much of our land covered by ice more than a mile thick. The memory of these events is built into the genetic plasticity of the species on this planet. IPCC forecasts are for changes to occur faster than evolution is considered to occur, so impacts will be determined by this plasticity from past experiences and the resiliency of affected organisms to find suitable habitats. However, in the ocean, I believe natural climatological variation has greater amplitude and speed, making projected changes less significant.
In the oceans, major climate warming and cooling and pH (ocean pH about 8.1) changes are a fact of life, whether it is over a few years as in an El NiĖo, over decades as in the Pacific Decadal Oscillation or the North Atlantic Oscillation, or in a few hours as a burst of upwelling (pH about 7.59-7.8) appears or a storm brings acidic rainwater (pH about 4-6) into an estuary. Natural, clean rainwater is over 100 times more acidic than ocean surface water and upwelling seawater is about the same as modeled climate scenarios (IPCC: 7.76-7.86).[4] It is noteworthy that these IPCC projections closely match the center of upwelling waters, the most productive areas of the ocean. In these areas, the supply of CO2, mixed with sunlight and nutrients, causes a bloom of algae that raises pH to 8.5 on its edges, an increase in alkalinity of over 200%[18], and creates the most productive fisheries on earth.
Despite severe and abrupt ocean climate changes in terms of currents, temperatures, salinity, pH, and other parameters, the biology changes rapidly to the new state in months or a couple years. These changes far exceed the changes expected with human-induced climate change and occur much faster. The estimated 0.1 change in alkalinity since 1750 and the one degree F. temperature rise since 1860 are but noise in this rapidly changing system. Sea level has been inexorably rising since the last glaciation lost its grip a mere 10,000 years ago. It is only some few thousand years since trees grew on Georges Bank and oysters flourished on its shores. Their remains still come up in dredges and trawls in now deep water, with the oysters looking like they were shucked yesterday. In the face of all these natural changes, and those we are here to consider, some species flourish while others diminish.
I do not know whether the earth is going to continue to warm, or that having reached a peak several years ago, we are at the start of a cooling cycle that will last several decades or more. I think carbon-based fuels will continue to increase in price and become scarcer as reserves are depleted even though I am an optimist about our technological advances in helping us find and exploit additional reserves. Nevertheless, our consumption is more likely to fall than to rise. In any case, I am optimistic about our ability to deal with the consequences.
The most important approach in determining the impact of CO2 on the oceans is to examine what happened during past times. The world has been down this path before and all the existing genera, and many species, endured. It has often been a difficult journey, with volcanism, meteoroid collisions, severe ice ages, and great heat, with many of these events causing mass extinctions. The ancestors of these animals were on Earth long before humans. They are the survivors of great disasters. The memory of these difficult times is in their genetic makeup. Adaptation will be swift, if needed.
With no laboratory or observational evidence of biological disruption, I see no economic disruption of commercial and recreational fisheries, nor harm to marine mammals, sea turtles or any other protected species. Whichever response the US takes, our actions should be prudent. Our fishing industry, maritime industry and other users of the ocean environment compete in a world market and are vulnerable in many ways to possible governmental actions to reduce CO2 emissions. We already import most of our seafood and many of the nations with which we compete do not need further advantages. Our research should focus on those ecosystem linkages we need to understand in order to wisely manage our fisheries, and conserve our protected species.
Our research should focus on understanding those ecosystem linkages needed to wisely manage our fisheries, and conserve our protected species. This includes research to explore further the possible acidification effects, as wisely envisioned with the funds recently made available to NOAA.
References: